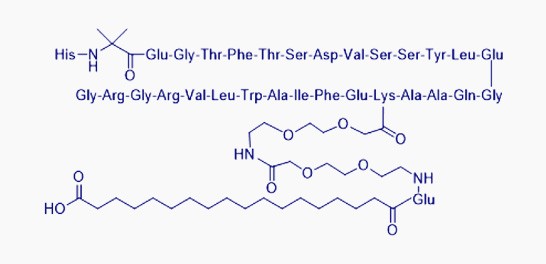
Semaglutide
四川多瑞药业
2025-05-22
US FDA Registration No.: 043251
Indication: Type Ⅱdiabetes, Obesity
Features:
Purity ≥ 99.0%, individual impurity ≤ 0.1%
Meets the quality requirements for oral tablets and injections
Status: Validation Production Complete
Batch Size: 15kg
Prev:
TirzepatideNext:
Retatrutide