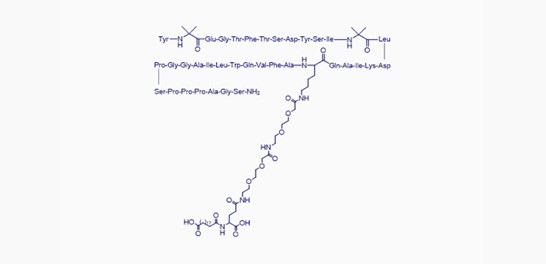
Tirzepatide
四川多瑞药业
2025-05-22
US FDA Registration No.: 043257
Indication: Type Ⅱdiabetes, Obesity
Feature: Dual-target GLP-1R/GIPR dual agonist
Batch Size: 15kg
Prev:
Lanreotide AcetateNext:
Semaglutide四川多瑞药业
2025-05-22

US FDA Registration No.: 043257
Indication: Type Ⅱdiabetes, Obesity
Feature: Dual-target GLP-1R/GIPR dual agonist
Batch Size: 15kg
Prev:
Lanreotide AcetateNext:
Semaglutide